Oddelenie syntézy a charakterizácie polymérov
The aim of the department is to cover completely the expertise in synthesis of polymers using various polymerization techniques including free radical, reversible-deactivation radical and anionic polymerizations. The second object of synthesis represents preparation, spectral characterization and mainly utilization of different fluorescence probes for characterization of polymer microstructure and for polymerization study mechanism. Modification of polymers by grafting, crosslinking and functionalizations and preparation of polymeric and inorganic nanoparticles and hybrids represents another part of synthetic direction. Prepared as well as comercial polymers are characterised by spectral methods (UV-VIS, FTIR, Fluorescence and Raman spectroscopy), thermal analysis (DSC, TGA, chemiluminescence, thermal and photo stability, flammability) and their molar characteristics by advanced HPLC techniques. Electron spin resonance (ESR) and positron annihilation lifetime spectroscopy (PALS) techniques are used for the microscopic structural-dynamic characterization of various pure and composed organic materials.

1. Study and development of RDRP and synthesis of functional polymers
Photochemically induced reversible-deactivation radical polymerization
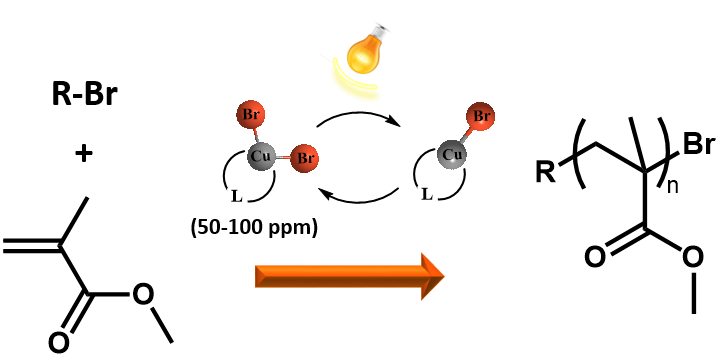
Development of RDRP is focused on use of photochemistry in initiation and propagation steps of polymerizations. Recently a new variation of atom transfer polymerization technique using only 50-100 ppm of stable complexes of higher oxidation state copper halides as a catalyst was developed and is further studied. In situ reduction of the catalyst can be achieved simply by UV or visible light. The advantage of the photochemically induced ATRP (photoATRP) is the synthesis of well defined polymers at ambient temperature while irradiation significantly increases also the rate constant of polymerization.
In addition to copper halides various other copper compounds such as, low-cost and widely available CuSO4·5H2O or various organic copper compounds (copper acetate, copper triflate, copper acetylacetonate, etc) can be also used as a catalysts in photoATRP. The polymerization rate, as well as control over the molar mass and dispersity of poly(methyl methacrylate), is nearly the same for all of the copper catalysts because in each case the polymerization is controlled by CuBr/CuBr2/L catalytic system formed in situ after the photochemical reduction of the investigated copper catalysts.
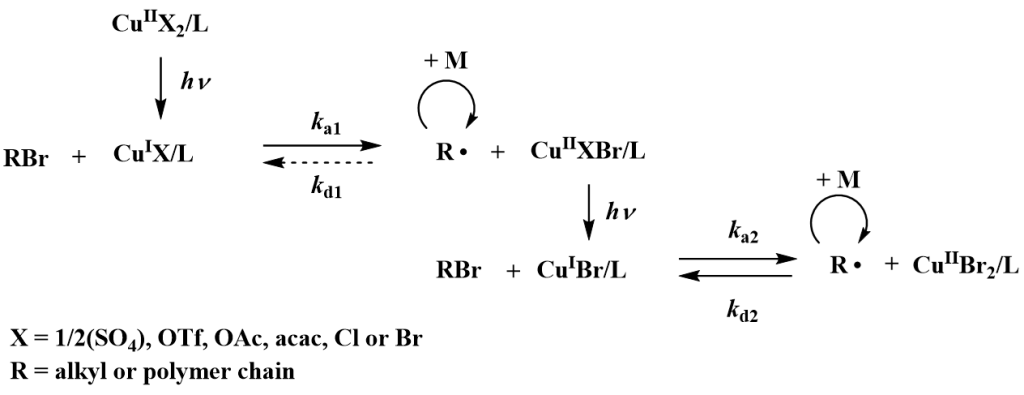

PhotoATRP is investigated also in the presence of a limited amount of air. It is expected that first there are cycles of oxidation of CuBr/L by reaction with oxygen and photochemical regeneration of CuBr/L. Subsequently after consumption of oxygen in the system the CuBr/L activates the alkyl halide to initiate the polymerization of MMA. The induction period before starting the polymerization can be shortened by using approximately a 4-fold excess of the TPMA ligand with respect to the copper catalyst. Thus the photoATRP as well as the chain extension polymerization can be successfully performed without the necessity of degassing monomers and solvents. In comparison with ARGET or ICAR ATRP, which can also be run under a limited amount of oxygen, the photoATRP does not need additional chemicals such as reducing agents or sources of radicals. The photoATRP system can have tremendous importance from an industrial point of view because costly, time consuming procedures of removing oxygen from the polymerization mixture can be avoided without losing control over the molecular characteristics of the final PMMA.The research is also focused on development of new organocatalysts for photoATRP and new initiators for photochemically mediated RDRP using photoactive nitroxides and alkoxyamines.
Related recent publications:
- J. Mosnacek, A. Andicsová-Eckstein, K. Borska „Ligand Effect and Oxygen Tolerance Studies in Photochemically Induced Copper Mediated Reversible Deactivation Radical Polymerization of Methyl Methacrylate in Dimethyl Sulfoxide”, Polym. Chem., Vol. 6, p. 2523-2530 (2015).
http://pubs.rsc.org/en/Content/ArticleLanding/2015/PY/C4PY01807A#!divAbstract - J. Mosnáček, A. Kundys, A. Andicsová „Reversible-deactivation radical polymerization of methyl methacrylate induced by photochemical reduction of various copper catalysts”, Polymers, Vol. 6 (10), p. 2862-2874 (2014).
http://www.mdpi.com/2073-4360/6/11/2862 - J. Mosnacek, M. Ilcikova „Photochemically Mediated Atom Transfer Radical Polymerization of Methyl Methacrylate Using ppm Amounts of Catalyst”, Macromolecules, Vol. 45 (15), p. 5859–5865 (2012).
http://pubs.acs.org/doi/abs/10.1021/ma300773t
Study of livingness and initiation efficiency of various RDRP techniques
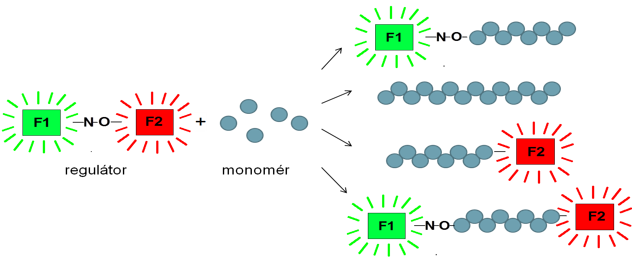
In comparison with living anionic polymerization, termination cannot be entirely avoided in reversible-deactivation radical polymerizations (RDRP, also known as controlled radical polymerizations), so these systems are never living at a level of ionic polymerizations. Study of RDRP is focused on studies of initiation efficiency and livingness of RDRP in dependency on type of polymerization technique and polymerization conditions. The livingness is studied using LC LCD technique of liquid chromatography enabling detection of even very small amount of homopolymer in block copolymers, or using fluorescent initiators and subsequent determination of concentration of fluorescent groups of the initiator bonded in the polymer chains by UV and/or fluorescent spectroscopy. Also combination of these two methods can be used by incorporation of a fluorescent detector to the GPC or HPLC system.
Related recent publications:
- Š. Chmela, J. Kollár, Ľ. Hrčková, „Fluorescent dye-labeled TIPNO type regulator for nitroxide mediated reversible-deactivation radical polymerization”, J. Photochem. Photobiol. A Chem., Vol. 307–308, p. 123 – 130 (2015).
http://www.sciencedirect.com/science/article/pii/S1010603015001392
2. Inorganic and carbon (nano)particles and hybrids
Smart biodecorated hybrid nanoparticles
The hybrid nano(bio)materials, namely quatum dots-, carbon nanotubes- and noble metal nanoparticles- based nanomaterials that have been recently explored, are used for sensing, imaging and therapeutic applications.
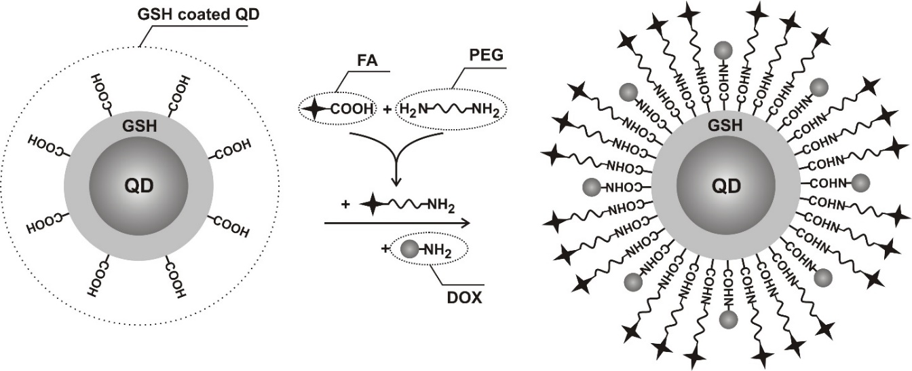
The enhanced cellular drug delivery to cancer cell lines via nanoconjugates revealed that PEG-covered gold nanoparticles, quantum dots, carbon nanotubes, are an effective tool for transporting and delivering biodecorated nanomaterials-bassed drugs. Biodecorated nanoparticles provide opportunities for designing various types of therapeutics with novel properties that are not possible to obtain with traditional therapeutics.
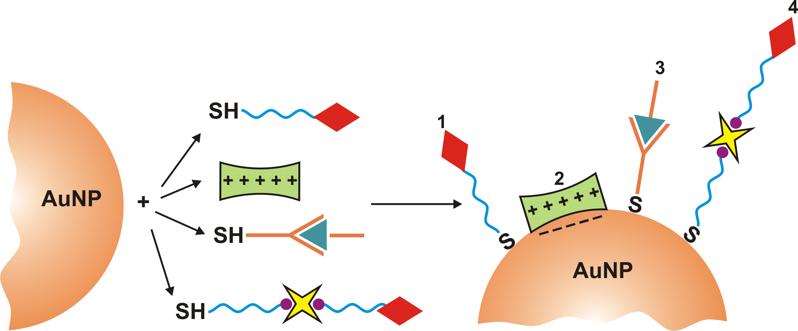
Related recent publications:
- I.Capek, „DNA Engineered Noble Metal Nanoparticles: Fundementals and State-of-the-Art of Nanotechnology“, Wiley&Sons, Scrivener Publishing USA, Ed. I. Capek, 2015, 1-656 p.
- I. Capek, „On Biodecorated Gold Nanoparticles Distributed within Tissues and Cells“, J. Nanomed. Res., Vol. 2 (2), Art.No. 00021, p. 1-10 (2015), DOI: 10.15406/jnmr.2015.02.00021
- I. Capek, „Plasmonic Nanoparticles and Their Conjugates: Preparation, Optical Properties and Antimicrobial Activity“, J. Nanotech. Mater. Sci., Vol. 2 (1), p. 1-18 (2015).
Polymeric hybrids prepared using surface initiated polymerizations and/or grafting onto methods
Good dispersion and homogeneous distribution of nanofillers in polymer matrices is the key factor for effective transfer of nanofiller properties to final polymer material at low nanofiller concentrations. Modification of CNT using surface initiated ATRP is performed in order to improve CNTs dispersion and/or obtain preferential interactions or even preferential localization of CNTs in one phase of triblock thermoplastic elastomers, such as polystyrene-block-polyisoprene-block-polystyrene or poly(methyl methacrylate)-block-poly(butyl acrylate)-block-poly(methyl methacrylate) (PMMA-b-PBA-b-PMMA).
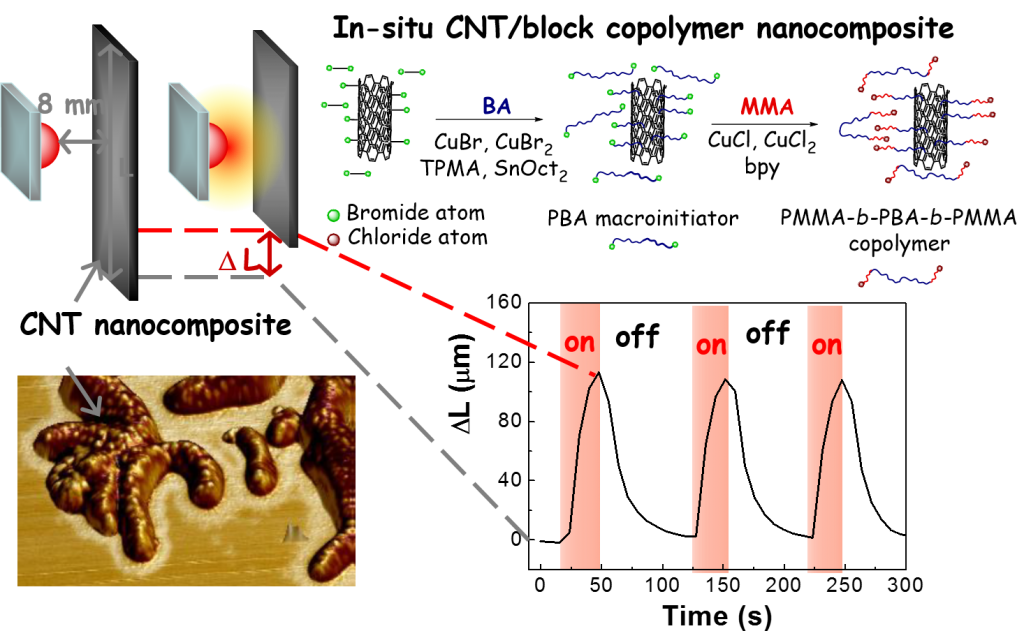
The specific interactions between the filler and individual blocks are investigated by DMA and characterized also based on calculated activation energy of glass transitions of the individual phases. Surface modification of nanofillers in one step during synthesis of polymer matrix can provide composites with well dispersed nanofillers. CNT-g-PBA-b-PMMA / PMMA-b-PBA-b-PMMA nanocomposite prepared using this method showed significant improvement of viscoelastic properties in the wide range of temperatures (from 0 to 240 °C) and high photoactuation ability with fast and reversible response.
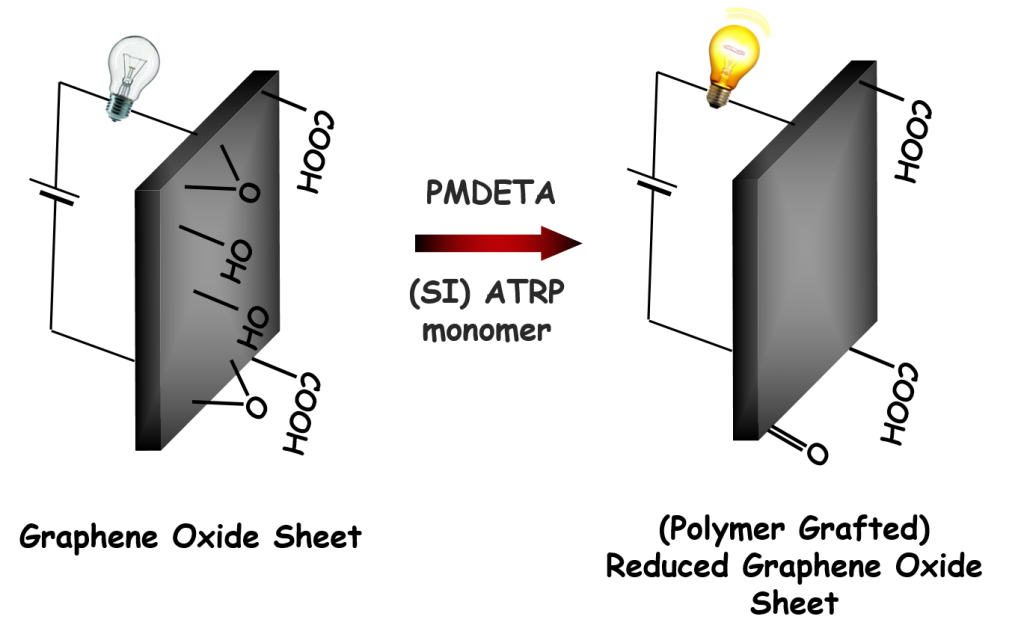
Synthesis of conductive hybrids and/or composites is investigated by in situ reduction of graphene oxide (GO) during (surface initiated) atom transfer radical polymerization. It was proven that tertiary amines, commonly used in ATRP as a ligands in the copper catalyst system, are responsible for the reduction of GO during ATRP and no further reducing agent is needed. Thus, the tertiary amine is involved in two separate competitive processes – catalysis of the polymerization and GO reduction. Using sufficient amount of a ligand is crucial to perform ATRP in the presence of GO. Using excess tertiary amine, compared to copper catalyst, can increase both the rate of polymerization and the reduction of GO. The final conductivity of the reduced GO hybrids can be finely tuned by adjusting the experimental conditions, as was successfully applied in preparation of reduced GO-based electromagnetic suspensions.
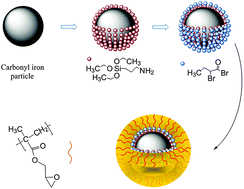
Surface initiated polymerizations are used for modification of inorganic (nano)particles for improvement of their stability in solutions for various applications, such as electro or magnetorheology.
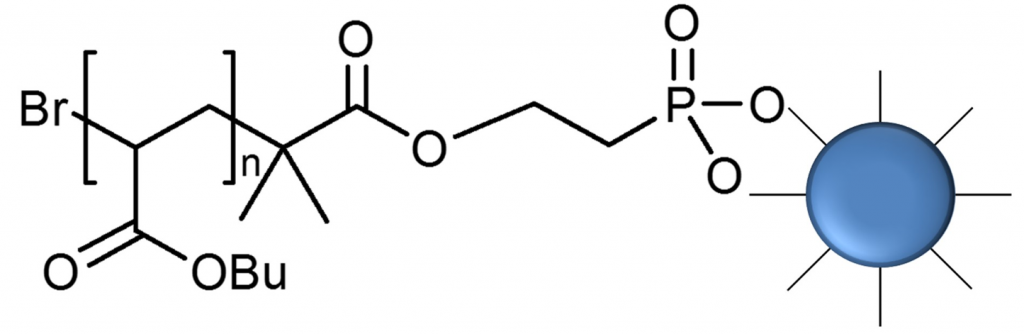
Design of iron oxide nanoparticles grafted with polymeric surfactants for chemiresistors. In this research, iron oxide nanoparticles (IONPs) were synthesized by thermal decomposition and reduction of Fe(acac)3, in the presence of oleic acid and oleylamine as surfactants. Several alkyl halide initiators containing either dopamine or phosphonic acid funcionalities were prepared and used for preparation of well-defined polymers using atom transfer radical polymerization (ATRP). The effect of polymer structure (polybutylacrylate and polystyrene) and the length of polymer chain of synthesized polymeric surfactants were investigated. In addition to polymer attachment onto the IONPs surface by “grafting onto” approach, also “grafting from” approach was applied. Well dispersed nanoparticles coated with various polymeric surfactants were purified by repeated cycles of redispersion/centrifugation. Dynamic light scattering analysis (DLS) was used to evaluate the size distribution profile and size of obtained nanoparticles. The diameter of oleyl-chains coated iron nanoparticles increased from 8 nm up to 15 – 20 nm after coating with polymer chains, depending on the nature and the molar mass of the employed polymeric surfactant.
Related recent publications:
- M. Ilčíková, M. Danko, M. Doroshenko, M. Mrlík, K. Csomorová, M. Šlouf, D. Chorvát, K. Koynov, J. Mosnáček, „Visualization of carbon nanotubes dispersion in composite by using confocal laser scanning microscopy“, Eur. Polym. J., Vol. 79, p. 187-197 (2016).
http://www.sciencedirect.com/science/article/pii/S0014305716300714 - M. Mrlík, M. Ilčíková, T. Plachý, V. Pavlínek, Z. Špitalský, J. Mosnáček, „Graphene oxide reduction during surface-initiated atom transfer radical polymerization of glycidyl methacrylate: Controlling electro-responsive propertie“, Chem. Eng. J., Vol., 283, p- 717-720 (2016)
http://www.sciencedirect.com/science/article/pii/S1385894715010839 - M. Cvek, M. Mrlík, M. Ilčíková, J. Mosnáček, V. Babayan, Z. Kuceková, P. Humpolíček, V. Pavlínek, „The chemical stability and cytotoxicity of carbonyl iron particles grafted with poly(glycidyl methacrylate) and the magnetorheological activity of their suspensions“, RSC Adv., Vol. 5, p. 72918-72824 (2015).
http://pubs.rsc.org/en/Content/ArticleLanding/2015/RA/c5ra11968e#!divAbstract - M. Ilčíková, M. Mrlík, T. Sedláček, M. Doroshenko, K. Koynov, M. Danko, J. Mosnáček, „Tailoring of viscoelastic properties and light-induced actuation performance of triblock copolymer composites through surface modification of carbon nanotubes“, Polymer, Vol. 72, p. 368-377 (2015).
doi:10.1016/j.polymer.2015.03.060 - M. Cvek, M. Mrlik, M. Ilcikova, T. Plachy, M. Sedlacik, J. Mosnacek, V. Pavlinek „A facile controllable coating of carbonyl iron particles with poly(glycidyl methacrylate): a tool for adjusting MR response and stability properties”, J. Mater. Chem. C, Vol. 3, p. 4646 (2015).
http://pubs.rsc.org/en/content/articlelanding/2015/tc/c5tc00319a#!divAbstract - M. Ilčíková, M. Mrlík, Z. Špitalský, M. Mičušík K. Csomorová, V. Sasinková, A. Kleinová, J. Mosnáček „A tertiary amine in two competitive processes: reduction of graphene oxide vs. Catalysis of Atom Transfer Radical Polymerization”, RSC Adv., Vol. 5, p. 3370–3376 (2015).
http://pubs.rsc.org/en/content/articlelanding/2015/ra/c4ra12915f#!divAbstract/a> - M. Ilčíková, M. Mrlík, T. Sedláček, M. Šlouf, A. Zhigunov, K. Koynov, J. Mosnáček, „Synthesis of photoactuating acrylic thermoplastic elastomers containing diblock copolymer-grafted carbon nanotubes“, ACS Macro Lett., Vol. 3, p. 999-1003 (2014).
http://pubs.acs.org/doi/ipdf/10.1021/mz500444m - M. Ilčíková, J. Mosnáček, M. Mrlík, T. Sedláček, K. Csomorová, K. Czaniková, I. Krupa, „Influence of surface modification of carbon nanotubesoninteractions with polystyrene-b-polyisoprene-b-polystyrene matrix and its photo-actuation properties“, Polym. Adv. Technol., Vol. 25, p. 1293-1300 (2014).
DOI: 10.1002/pat.3324 - M. Ilčíková, M. Mrlík, T. Sedláček, D. Chorvát, I. Krupa, M. Šlouf, K. Koynov, J. Mosnáček, „Viscoelastic and photo-actuation studies of composites based on polystyrene-grafted carbon nanotubes and styrene-b-isoprene-b-styrene block copolymer“, Polymer, Vol. 55, p. 211-218 (2014).
doi:10.1016/j.polymer.2013.11.031 - S. S.Texeira, M. P. F. Graca, M. Dionisio, M. Ilčíková, J. Mosnáček, Z. Špitalský, I. Krupa, L. C. Costa, „Self-standing elastomeric composites based on lithium ferrites and their dielectric behavior“, J. App. Phys., Vol. 116, p. 224102 (2014).
http://scitation.aip.org/content/aip/journal/jap/116/22/10.1063/1.4903735 - M. Mrlík, M. Ilčíková, M. Sedlačík, J. Mosnáček, P. Peer, P. Filip, „Cholesteryl-coated carbonyl iron particles with improved anti-corrosion stability and their viscoelastic behaviour under magnetic field“, Colloid Polym. Sci., Vol. 292, p. 2137-2143 (2014).
http://link.springer.com./article/10.1007%2Fs00396-014-3245-5 - M. Mrlík, M. Ilčíková, V. Pavlínek, J. Mosnáček, P. Peer, P. Filip, „Improved thermooxidation and sedimentation stability of covalently-coated carbonyl iron particles with cholesteryl groups and their magnetorheology“, J.Colloid Inter. Sci., Vol. 396, p. 146-151 (2013).
http://www.sciencedirect.com/science/article/pii/S0021979713000659 - M. Brzezinski, M. Boguslawska, M. Ilcikova, J. Mosnacek, T. Biela „Unusual Thermal Properties of Polylactides and Polylactide Stereocomplexes Containing Polylactide-Functionalized Multi-Walled Carbon Nanotubes”, Macromolecules, Vol. 45, p. 8714-8721 (2012).
http://pubs.acs.org/doi/abs/10.1021/ma301554q - Z. Spitalsky, M. Danko, J. Mosnacek „Preparation of Functionalized Graphene Sheets”, Curr. Org. Chem., Vol. 15, p. 1133-1150 (2011).
DOI: 10.2174/138527211795202988
3. Polymeric (nano)particles, (hydro)gels and (nano)fibres
Polymer and composite nanoparticles prepared using heterogeneous polymerizations
The synthesis of nanocomposite polymer and copolymer particles in miniemulsions is studied. Research efforts are focused on the factors affecting kinetics and mechanism of radical polymerization of convenctional and nonconvenctional monomers in both the direct and inverse miniemulsion. We investigate the locus of initiation, the mechanism of particle nucleation, deactivation of growing radicals within the polymer particles and the transport of monomer within the multiphase reaction system. Emphasis is given to understanding the principles of regulation of the reactivity of radicals and monomers and a phenomenological study of the processes responsible for the formation and changes in pure polymer and composite nanoparticles and disperse systems. The objective is to discover ways to prepare conventional and nonconventional polymer latexes and composite nanomaterials with predetermined chemical and physical properties for conventional and special applications.
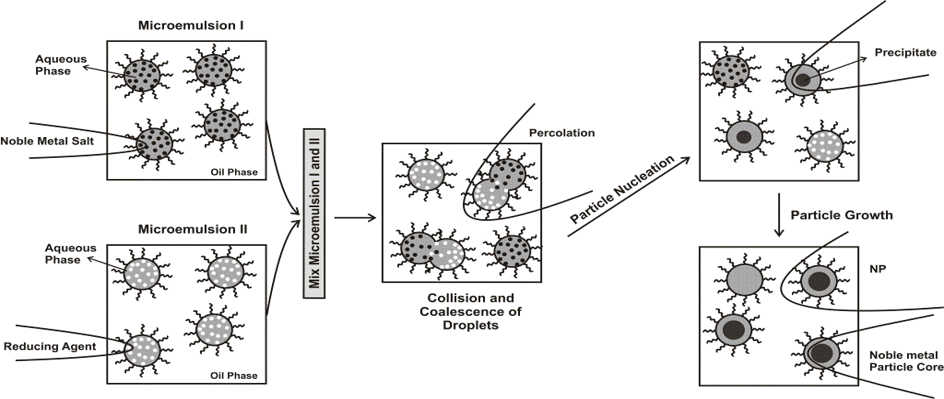
In addition, 0D and 1D water- and oil-soluble metal nanoparticles are prepared by classical microemulsion approach and by high-temperature approach.

From tulips to polymeric nanoparticles. MBL was used as a monomer for the preparation of nanoparticles via heterogeneous polymerization process. The results are presented for the surfactant as well as surfactant-free polymerization of MBL with ionic, water soluble initiator potassium persulfate. The influence of polymerization technique (batch or semibatch), type of surfactant, surfactant concentration and ionic strength of the aqueous phase on molar mass and size of nanoparticles was studied. As a surfactant, sodium dodecyl sulphate, Tween 80 and monomer SHMB prepared by saponification of MBL were examined. The ionic strength was generated by addition of NaCl electrolyte. The size of the particles increases gradually with increasing ionic strength of the aqueous phase. The weight average molecular weights and molecular weight distributions were determined by size exclusion chromatography (SEC). Dynamic light scattering (DLS) was used to evaluate the size distribution profile and size of PMBL particles. According to experimental conditions nearly monodisperse polymer particles of 0.15 to 0.60 µm were produced.
Related recent publications:
- I. Capek, „On photoinduced polymerization of acrylamide“, Design. Monom. Polym., Vol. 17 (4), p. 356-363 (2014).
- M. C. Corobea, I. Capek, R. Ianchis, D. Donescu, R. Somoghi, M. Ghiurea, C. L. Nistor, V. Purcar, L. O. Cinteza, C. Radovici, G. Prodan, „Silica nanowires obtained on clay mineral layers and their influence on mini-emulsion polymerisation“, Appl. Clay Sci., Vol. 95, p. 232-242 (2014).
- I. Capek, „On photoinduced miniemulsion polymerization of butyl acrylate with clay“, Design. Monom. Polym., Vol. 15 (4), p. 345-355 (2012).
Polymeric superabsorbent hydrogels
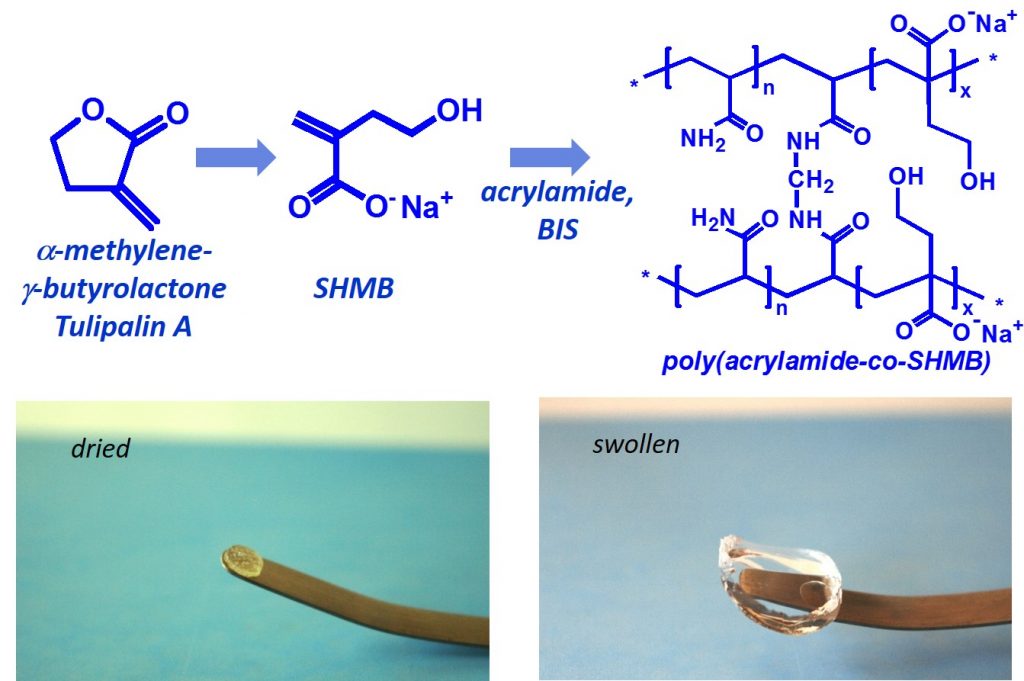
Polymers derived from nature were synthesized as a promising material with superabsorbent properties. α-Methylene-γ-butyrolactone (MBL), also known as Tulipalin A, is fungitoxic substance isolated mainly from tulips. In our research we focused on synthesis and properties of new superabsorbent polymers derived from hydrolyzed MBL – 4-hydroxy-2-methylenebutanoate (SHMB). Copolymerization of SHMB with acrylamide (AM) at various ratios in the presence of crosslinker yielded hydrogels with superior degree of swelling and comfortable handling. The effect of chemical composition (AM : SHMB ratio), the concentration of monomers in water and amounts of crosslinker were investigated. Hydrogels showed equilibrium degree of swelling in the range of 13,000 – 82,000%. Swelling capacity significantly increased with increased amount of SHMB. The viscoelastic characteristics of the hydrogels were significantly influenced by both the monomers ratio and crosslinker content.
Related recent publications:
- J. Kollár, M. Mrlík, D. Moravčíková, Z. Kroneková, T. Liptaj, I. Lacík, J. Mosnáček „Tulips: A Renewable Source of Monomer for Superabsorbent Hydrogels”, Macromolecules, 2016, 49 (11), 4047-4056.
http://pubs.acs.org/doi/abs/10.1021/acs.macromol.6b00467
Polymeric (nano)fibres and fiber composites prepared by electrospinning
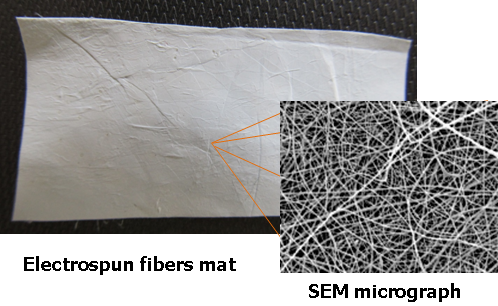
Electrospinning is often used method, due to its versatility and cost-effectiveness that allows the production of functional nano-fibers from various materials including polymers, polymer blends, biopolymers, sol-gels and composite materials. Prepared fibers have several remarkable characteristics such as large specific surface area, pore size in nano-range, unique physical properties and flexible design, which allows chemical and physical binding of specific additives to the nano-fibers. In our experiments we studied the preparation and modification of electrospun fiber by specific “click” reaction to obtain particular materials for medicine and agrochemistry dependent on the active component. The main goal of this research is to prepare the functional electrospun fibers and immobilize the active molecules in the structure of fibers or on their surface for using in special applications. Fibrous mats are prepared from biodegradable polymers such as PVA, PLA, PCL, PHB, copolymers and natural polymers such as cellulose or silk.
4. Synthesis, spectral properties and application of photoactive compounds
Fluorescent probes for study of polymers structures, microenvironment, processes in polymer fims and behaviour of polymers in solutions
Main interest in this field is focused to the synthesis and spectral characterization of novel multifunctional fluorescence probes suitable for the characterization of microenvironment of complex polymer systems dynamic processes in this systems and their behavior in radical processes. The attention is also focused on general study of influence of paramagnetic center of stabile radical to the quenching of fluorescence of different fluorophores.
Related recent publications:
- M. Danko, P. Kasák, P. Hrdlovič, „The interactions of probes based on substituted pyrene derivatives in polymer matrices; spectral study“, J. Photochem Photobiol. A: Chem., Vol. 307, p. 79-87 (2015). doi: 10.1016/j.jphotochem.2015.04.008M
- J. Kollár, S. Chmela, P. Hrdlovič, „Spectral properties of bichromophoric probes based on pyrene and benzothioxanthene in solution and in polymer matrices“, J. Photochem. Photobiol A Chem., Vol. 270, p. 28 – 36 (2013).
- C. Kósa, M. Danko, P. Hrdlovič, „Preparation and Spectral Characterization of Fluorescence Probes Based on 4-N,N-Dimethylamino Benzoic Acid and Sterically Hindered Amines“, J. Fluorescence, Vol. 22 (5), p. 1371-1381 (2012), DOI:10.1007/s10895-012-1076-7
- M. Danko, E. Szabo, P. Hrdlovic, „Synthesis and Spectral Characteristics of Fluorescent Dyes Based on Coumarin Fluorophore and Hindered Amine Stabilizer in Solution and Polymer Matrices“, Dyes Pigm., Vol. 90 (2), p. 129-138 (2011), DOI: 10.1016/j.dyepig.2010.12.006
- M. Danko, P. Hrdlovič, Š. Chmela, „The photolysis in polymer matrices of dyes containing a benzothioxanthene chromophore linked with a hindered amine“, Polym. Degrad. Stab., Vol. 96, p. 1955-1960, (2011). doi:10.1016/j.polymdegradstab.2011.08.006
New electro- and photo-active derivatives for electronic devices and other applications
Synthesis and complex spectral characterization of novel electro- and photo-active derivatives and fluorescent labels with targeted spectral properties and their application in photonic and sensoric chemistry represents one of the major topic of the group with partners form SAS or abroad co-operations. In modern material research of novel organic molecules suitable for the application in electronics is connected with the tuning of the optical and electrochemical properties, e.g. using the suitable combination of chromophoric moieties. The defined combination of fluorene, perylene, phthalimide, tetrahydroquinoline, naphthyl phenothiazine and oxadiazole units represent nowadays an innovative basis for this material research. As a part of this research, synthesis of reactive fluorescent labels for preparation of different inorganic/organic hybrid materials as layered silicates or graphite-based nanoparticles is focused as well.
Related recent publications:
- E. Kozma, G. Grisci, W. Mróz, M. Catellani, A. Eckstein-Andicsová, K. Pagano, F. Galeotti, “Water-soluble aminoacid functionalized perylene diimides: The effect of aggregation on the optical properties in organic and aqueous media“, Dyes and Pigments, Vol. 125, p. 201-209 (2016).
- E. Kozma, W. Mróz, F. Villafiorita-Monteleone, F. Galeottia, A. Andicsová-Eckstein, M. Catellani, C. Botta, „Perylene Diimide Derivatives as Red and Deep Red-Emitters for Fully Solution Processable OLEDs“, RSC Adv., Vol. 6, p. 61175-61179 (2016).
- M. Ilčíková, M. Danko, M. Doroshenko, A. Best, M. Mrlík, K. Csomorová, M. Šlouf, D. Chorvát Jr., K. Koynov, J. Mosnáček, Visualization of carbon nanotubes dispersion in composite by using confocal laser scanning microscopy. Eur. Polym. J., Vol. 79, p. 187-197 (2016). doi:10.1016/j.eurpolymj.2016.02.015
- P. Kasák, J. Mosnáček, M. Danko, I. Krupa, G. Hloušková, D. Chorvát, M. Koukaki, S. Karamanou, A. Economou, I. Lacík, A polysulfobetaine hydrogel for immobilization of a glucose-binding protein. RSC Advances, Vol. 6, p. 83890-83900 (2016). doi: 10.1039/c6ra14423c;
- S. Sas, M. Danko, K. Lang, J. Bujdák, „Photoactive hybrid material based on kaolinite intercalated with a reactive fluorescent silane“, Appl. Clay Sci., Vol. 108, p. 208-214 (2015), http://dx.doi.org/10.1016/j.clay.2015.02.031
- M. Danko, A. Andicsová, P. Hrdlovič, D. Račko, D. Végh, „Spectral Characteristics of Carbonyl Substituted 2,2’-Bithiophenes in Polymer Matrices and Low Polar Solvents“, Photochem. Photobiol. Sci., Vol. 12 (7), p. 1210 – 1219 (2013), doi: 10.1039/c3pp50049g
- M. Danko, A. Andics, C. Kosa, P. Hrdlovič, D. Vegh, Spectral Properties of Chalcone Containing Triphenylamino Structural Unit in Solution and in Polymer Matrices, Dyes Pigm., Vol. 92(3), p. 1257-1265, (2012). doi:10.1016/j.dyepig.2011.07.011
Photochemically active compounds for cross-linking and/or tailoring of degradability of polymers
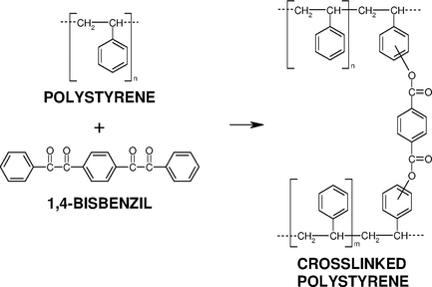
Special attention is devoted to the synthesis and evaluation of reactivity of photoactive compounds (phenyl ketones, 1,2-diketones) in synthetic (polystyrene, poly(methyl methacrylate), etc.) and biocompatible (poly(L-lactide), poly(-caprolactone), etc.) polymer matrices, photochemical in situ synthesis of free or pendant diacyl peroxides in polymer films, exploitation of diacyl peroxides for grafting and cross-linking and characterization of the final functional polymer materials. Crosslinking of polystyrene film with bis(dibenzoyl peroxides) formed from dibenzils by photoperoxidation with oxygen and visible light was studied too.
Related recent publications:
- I. Lukáč, B. Husár, C. Kósa, J. Faryová „Method for cross-linking of polymer films”. International application No. PCT/SK2014/050008 (WO2014209241)
- Cs. Kosa, M. Sedlacik, A. Fiedlerova, Š. Chmela, K. Borska, J. Mosnacek „Photochemically cross-linked poly(e-caprolactone) with accelerated hydrolytic degradation”, Eur. Polym. J., Vol. 68, p. 601–608 (2015).
- I. Lukáč, Cs. Kósa, B. Husár, „1,4-Bisbenzil: Visible-light- and heat-assisted crosslinking of polystyrene films”, Macromol. Chem. Phys., Vol. 215, p. 171 – 176 (2014).
- J. Mosnáček, K. Borská, M. Danko, I. Janigová, „Photochemically promoted degradation of poly(ɛ-caprolactone) film“, Mater. Chem. Phys., Vol. 140, p. 191-199 (2013), doi: 10.1016/j.matchemphys.2013.03.021
- J. Mosnacek, I. Lukac, M. Bertoldo, F. Ciardelli „Applicability of photochemically generated pendant benzoyl peroxides in both “grafting from” and “grafting to” techniques”, Chem. Papers, Vol. 67 (1), p. 9-17 (2013).
- B. Husár, N. Moszner, I. Lukáč, „Synthesis and photooxidation of styrene copolymer bearing camphorquinone pendant groups”, Beilst. J. Org. Chem., Vol. 8, p. 337 – 343 (2012).
- B. Husár, I. Lukáč, „Photooxidation of camphorquinone in polystyrene matrix”, J. Photochem. Photobiol. A Chem., Vol. 233, p. 189–193 (2011).
- Š. Chmela, A. Fiedlerová, I. Janigová, I. Novák, E. Borsig „Grafting of iPP powder with methacrylate monomers in water medium”, J. Appl. Polym. Sci., Vol. 119, p. 2750–2758 (2011).
5. Synthesis of polymers and polymeric materials from renewable monomers
New functional polymers from a renewable γ-butyrolactone monomers
Wide range of compounds containing γ‒lactone ring can be found or obtained after some process from various types of plants, while many of them are also biologically active. Our attention is paid to use some of that compounds, such as α‒methylene‒γ‒butyrolactone (MBL), γ‒methyl‒α‒methylene‒γ‒butyrolactone (MMBL), α-angelica lactone and β‒angelica lactone in synthesis new types of functional polymers. α-Methylene-γ-butyrolactone (MBL), known also as a Tulipalin A, is present in the form of glycoside (Tuliposide A) in tulips in relatively high concentrations (0.2–2 wt.% of the glycoside in fresh weight of various parts of tulips). In addition, MBL can be produced also from biomass sugar-based itaconic anhydride or by biosynthesis from pyruvate and acetyl coenzyme A.
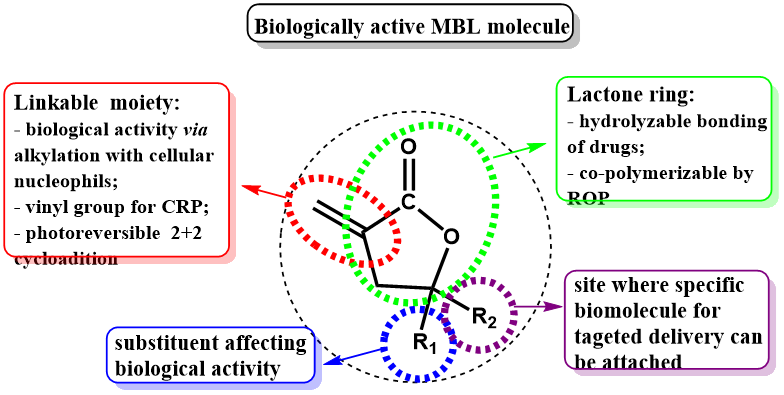
MBL consists of five-member lactone ring and exocyclic carbon-carbon double bond and therefore it can serve as dual monomer enabling both the radical polymerizations and ring opening copolymerizations.
Ring opening copolymerizations of angelica lactones and MBL with other lactones such as ε-caprolactone and L,L-dilactide are intensively studied under various polymerization conditions and using various catalysts. Final functional polyesters contain a double bonds along the chain, which can be further available for different types of post-functionalization.
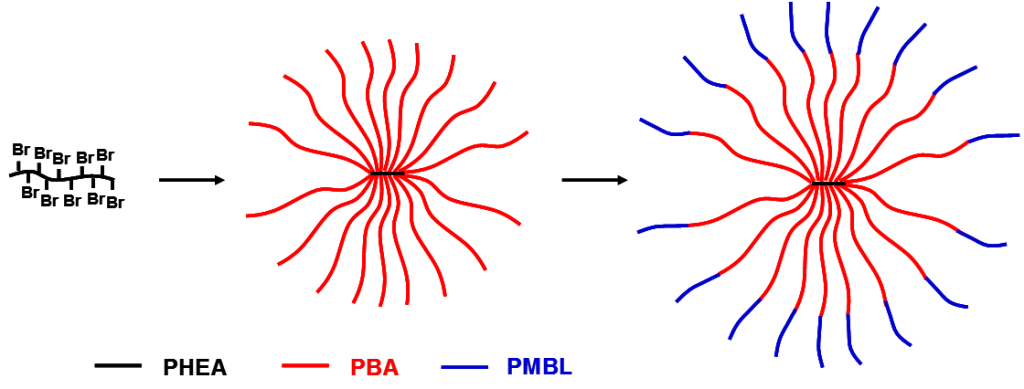
Reversible-deactivation radical polymerizations are used to functional (co)polymers with pendant butyrolactone ring for further functionalization. Block copolymers of MBL and butyl acrylate show properties of thermoplastic elastomers with high thermal stability and elastic properties remaining even at temperatures up to 350 °C.

Tulipalin A – monomer for the preparation of superabsorbent hydrogels. Polymers derived from nature were synthesized as a promising material with superabsorbent properties. α-Methylene-γ-butyrolactone (MBL), also known as Tulipalin A, is fungitoxic substance isolated mainly from tulips. In our research we focused on synthesis and properties of new superabsorbent polymers derived from hydrolyzed MBL – 4-hydroxy-2-methylenebutanoate (SHMB). Copolymerization of SHMB with acrylamide (AM) at various ratios in the presence of crosslinker yielded hydrogels with superior degree of swelling and comfortable handling. The effect of chemical composition (AM : SHMB ratio), the concentration of monomers in water and amounts of crosslinker were investigated. Hydrogels showed equilibrium degree of swelling in the range of 13,000 – 82,000%. Swelling capacity significantly increased with increased amount of SHMB. The viscoelastic characteristics of the hydrogels were significantly influenced by both the monomers ratio and crosslinker content.

From tulips to polymeric nanoparticles. MBL was used as a monomer for the preparation of nanoparticles via heterogeneous polymerization process. The results are presented for the surfactant as well as surfactant-free polymerization of MBL with ionic, water soluble initiator potassium persulfate. The influence of polymerization technique (batch or semibatch), type of surfactant, surfactant concentration and ionic strength of the aqueous phase on molar mass and size of nanoparticles was studied. As a surfactant, sodium dodecyl sulphate, Tween 80 and monomer SHMB prepared by saponification of MBL were examined. The ionic strength was generated by addition of NaCl electrolyte. The size of the particles increases gradually with increasing ionic strength of the aqueous phase. The weight average molecular weights and molecular weight distributions were determined by size exclusion chromatography (SEC). Dynamic light scattering (DLS) was used to evaluate the size distribution profile and size of PMBL particles. According to experimental conditions nearly monodisperse polymer particles of 0.15 to 0.60 µm were produced.
Related recent publications:
- J. Kollár, M. Mrlík, D. Moravčíková, Z. Kroneková, T. Liptaj, I. Lacík, J. Mosnáček „Tulips: A Renewable Source of Monomer for Superabsorbent Hydrogels”, Macromolecules, 2016, 49 (11), 4047-4056.
http://pubs.acs.org/doi/abs/10.1021/acs.macromol.6b00467 - A. Juhari, J. Mosnacek, J. A. Yoon, A. Nese, Koynov, T. Kowalewski, K. Matyjaszewski „Star-like Poly(n-butyl acrylate)-b-poly(alpha-methylene-gamma-butyrolactone) Block Copolymers for High Temperature Thermoplastic Elastomers Applications”, Polymer, Vol. 51 (21), p. 4806–4813 (2010).
http://www.sciencedirect.com/science/article/pii/S0032386110006919
6. Degradation, stabilization and flammability of polymers
Expertise includes:
- Thermal oxidation and ageing of materials
- Prediction of the remaining service life of the industrially produced polymers based on investigation of the life trajectory of the polymer material
- Synthesis and testing of photo- and thermostabilizers
- Ignitability a burning of polymers, characterization studies on cone calorimeter, model of ignition, burning and extinction of burning material
Tests of oxidation stability and of extent of degradation
Oxidation stability tests follow from Bolland Gee scheme: time or temperature evolution of concentration of hydroperoxides, DSC, thermogravimetry, chemiluminiscence, analytical determination of carbonyls.
Life trajectory of the polymer product polymers utilised practically have unknown residual stability due to unknown concentration of additives and modification procedure, points A,B,C and D represent the examples

Related recent publications:
- J. Rychlý, L. Matisová-Rychlá, K. Csomorová, „Degradation of plastics from the ResinKit as a model for the selection of polymers for artworks. Assessment by nonisothermal thermogravimetric analysis and chemiluminometry“, Polym. Degrad. Stab., Vol. 102, p. 105 – 111 (2014).
- J. Rychlý, L. Rychlá, A. Fiedlerová, S. Chmela, M. Hronec, „Thermally and UV initiated degradation of polypropylene in the presence of 2,5 bis(2-furylmethylene) cyclopentanone and heterogeneous distribution of hydroxides assessed by non-isothermal chemiluminescence in nitrogen“. Polym. Degrad. Stab., Vol. 108, p. 41-47 (2014).
- J. Rychlý, L. Matisová-Rychlá, K. Csomorová, „Reprint of degradation of plastics from the ResinKit as a model for the selection of polymers for artworks. Assessment by nonisothermal thermogravimetric analysis and chemiluminometry“. Polym. Degrad. Stab., 2014, vol. 107, p. 191-197.
Thermostability of phase change materials based on linear low-density polyethylene, paraffin wax and expanded graphite
PCMs with improved thermal conductivity, based on linear low-density polyethylene (LLDPE), paraffin wax with a melting point of approximately 42°C and expanded graphite are prepared and their physical behavior is investigated.
Heat transport is usually enhanced in PCMs by incorporating thermally conductive fillers. However, problems arise when attempting to minimize the filler content and maximize the paraffin content to optimize the functionality and maintain a compact form. Thus, these three components must be optimized. Expanded graphite (EG) has been extensively used over the past few years to improve thermal conductivity of particularly paraffin-based PCMs.
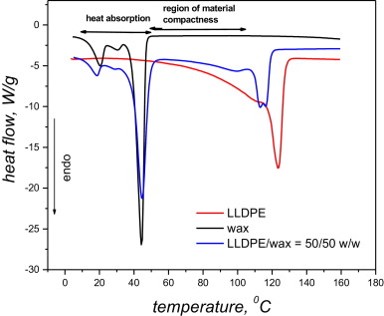
An estimate of the total heat energy that can be reversibly absorbed or released by the designed materials was determined using Differential Scanning Calorimetry (DSC). The improvement in the thermal conductivity of the polymeric materials was obtained by incorporating expanded graphite into the blends. Significant amounts of wax are leached from the samples, and higher wax content also results in more wax leaching. Graphite significantly reduces the wax loss from the samples.
Related recent publications:
- I. Krupa, Z. Nogellova, Z. Špitalský, M. Malíková, P. Sobolčiak, H. Abdelrazeq, M. Ouederni, M. Karkri, I. Janigova, M. AlMaadeed „Positive influence of expanded graphite on the physical behavior of phase change materials based on linear low-density polyethylene and paraffin wax“, Thermochim. Acta, doi:10.1016/j.tca.2015.06.028
- L. F. Cabeza, C. Barreneche, I. Martorell, L. Miró, S. Sari-Bey, M. Fois, H. Paksoy, N. Sahan, R. Weber, M. Constantinescu , E. Anghel, M. Malikova, I. Krupa, M. Delgado, P. Dolado, P. Furmanski, M. Jaworski, T. Haussmann, S. Gschwander, A. Fernández, „Unconventional technologies available for phase change materials (PCM) characterization. Part 1. Thermophysical properties.” Renew. Sustain. Energy Rev., Vol. 43, p. 1399–1414 (2015).
- A. Fernández, A. Solé, J. Giró-Paloma, M. Martínez, M. Hadjieva, A. Boudenne, M. Constantinescu, El. Anghel, M. Malikova, I. Krupa, C. Peñalosa, A. Lázaro, H. Paksoy, K. Cellat, J. Vecstaudž, D. Bajare, B. Sumiga, B. Boh, T. Haussmann, S. Gschwander, R. Weber, P. Furmanski, M. Jaworski, L. Cabeza „Unconventional technologies used for phase change materials (PCM) characterization. Part 2. Morphological and structural characterization, physico-chemical stability and mechanical properties“, Renew. Sustain. Energy Rev., Vol. 43, p. 1415–1426 (2015).
Study of photochemical, hydrolytic and enzymatic degradation of polymers
Photochemical, hydrolytic and enzymatic degradation of biodegradable polymeric materials, such as polylactide-based blends and composites applicable in food packaging and agriculture is studied under laboratory as well as real agricultural conditions. Extent of degradation is investigated by UV-vis, FT IR, Raman spectroscopies, GPC, mechanical properties, DSC, TGA, mass decrease and carbon dioxide production.
Related recent publications:
- K. Borska, M. Danko, J. Mosnacek „Photodegradation and Photochemical Cross-linking of Polylactide”, Chem. Listy, Vol. 108, p. 1030-1039 (2014).
http://www.chemicke-listy.cz/docs/full/2014_11_1030-1039.pdf
Synthesis and testing of thermal and photochemical stabilizers
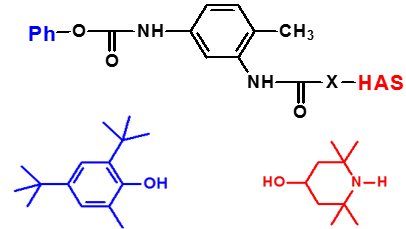
New types of thermal and photochemical stabilizers based on hindered phenols and hindered amines derivatives are synthesized and their stabilization efficiency is investigated. Special attention is paid to synthesis of higher molecular weight stabilizers and combined phenols / amines stabilizers with synergistic effect. Hyperbranched macrostabilizers with decreased leakage from the polymers also investigated in polyolefins as well as various types degradable polyesters. Comercially available antioxidants and UV absorbers are investigated in new types of polymers polymeric materials as well.
Related recent publications:
- G. Kasza, K. Mosnackova, A. Nador, Z. Osvath, T. Stumphauser, G. Szarka, K. Czanikova, J. Rychly, Š. Chmela, B. Ivan, J. Mosnacek „Synthesis of hyperbranched poly(ethyleneimine) based macromolecular antioxidants and investigation of their efficiency in stabilization of polyolefins”, Eur. Polym. J., Vol. 68, p. 609–617 (2015).
doi:10.1016/j.eurpolymj.2015.03.037 - J. Rychlý, K. Mosnáčková, L. Rychlá, Fiedlerová A., G. Kasza, A. Nádor, Z. Osváth, T. Stumphauser, G. Szarka, K. Czaniková, Š. Chmela, B. Iván, J. Mosnáček „Comparison of the UV stabilisation effect of commercially available processing stabilizer Irganox HP 136 with Irganox 1010 and synthesized hyperbranched phenolic antioxidants”, Polym. Degrad. Stab., Vol. 118, p. 10-16 (2015).
doi:10.1016/j.polymdegradstab.2015.04.007 - J. Rychly, L. Rychla, A. Fiedlerova, S. Chmela, M. Hronec, „Thermally and UV initiated degradation of polypropylene in the presence of 2,5 bis(2-furylmethylene) cyclopentanone and heterogeneous distribution of hydroperoxides assessed by non-isothermal chemiluminescence in nitrogen”, Polym. Degrad. Stab., Vol. 108, p. 41-47 (2014).
doi:10.1016/j.polymdegradstab.2014.05.022
Cone Calorimeter and Burning of Polymers
Determination of Heat Release Rate (HRR), ignition time, mass loss during combustion, quantitative determination of smoke density and other parameters
Related recent publications:
- J. Rychlý, M. Hudáková, L. Rychlá, I. Janigová, K. Csomorová, I. Chodák, „Magnesium hydroxide and magnesium oxide in oxidation and burning of polypropylene“, J. Sci. Res. Rep., Vol. 3 (6), p. 772-776 (2014).
- J. Rychlý, M. Hudáková, L. Rychlá, „Burning of thermally thin polyethylene mixtures. Cone calorimeter study“, J. Therm. Anal. Calorim., Vol. 115, p. 527 – 535 (2014).
Flammability of Smart Textiles Based on Expanded Graphite-Polymeric Nanocomposites
Smart textiles including electrically conducting textiles are developed in last decades due to many applications in medical, military, and sports, as well as in the industrial textile areas. Many of methods and coating techniques were used for manufacturing of conductive textiles, including conducting polymers, metals fibers incorporation, etc.
Conductive textiles prepared by surface coating with polymer composites containing expanded graphite were studied in our lab. The flammability, conductivity, thermostability, and contact angles were measured before and after washing of coated textiles with detergents.
Electrical conductivity of all untreated textiles was about 10-14 S/cm. The surface treatment with conducting suspension of graphite led to substantially higher values of conductivity. SEM pictures demonstrated the quality of surface treatment by polymeric matrix Axilat containing graphite. The coating remains preserved also after series of 5 cycle washing.
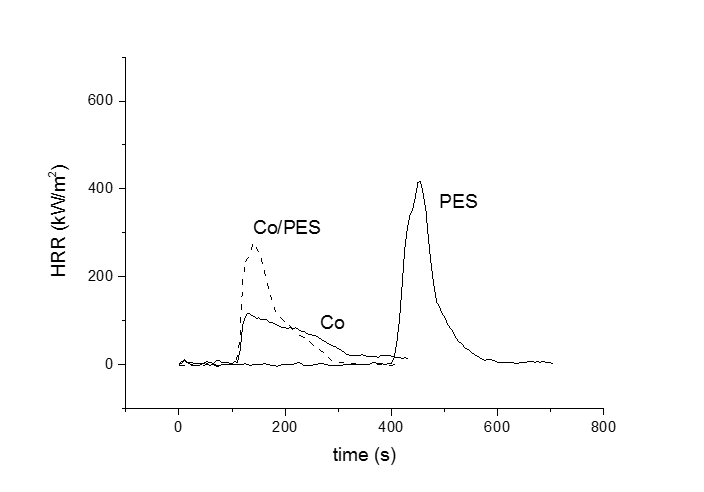
Flammability of textiles was investigated by the cone calorimeter. Parameters characterizing the burning processes as heat release rate, time to ignition, total oxygen consumed, total smoke released, effective heat of combustion as well as calculated value of maximum rate of heat emission (MARHE) were obtained. We can notice that the time to ignition increases in the order cotton = Co/PES < PES (Fig.). After ignition HRR increased to its maximal value for PES and Co/PES, between 290 and 500 kW/m2. The decay of the HRR which precedes the total consumption of the sample is rather fast. Burning behavior of cotton is different, it decreases less abrupt and is accompanied by long-term char glowing.
TGA results showed that washing lowered the onset temperature, the first step of degradation of washed samples probably due to using detergent SDS. The second step of TGA curves depended on the textile type. Textile treatment with polymeric matrices has an impact on thermal stability of washed samples – it lowers the onset of degradation, depending on the textile type. From studied textile treated PES samples showed the best thermostability.
7. Liquid chromatography research for effective separation of macromolecules
…under construction…
8. Structure and physico – chemical properties of polymers
Head of the Group: Josef Bartoš, D.Sc.
Members of the Group: Dr. Helena Švajdlenková, Dr. Miroslava Lukešová
The Profile of the Group:
The research activities of the Group of Structure and physico-chemical properties are focused on a basic research of the mutual relationships between the chemical structure of constituents, their physical structure and numerous physical and physico-chemical properties of various organic materials in various phase and physical states, especially of polymers, using a unique combination of the external probe techniques using electron spin resonance (ESR) and positron annihilation lifetime spectroscopy (PALS); the latter in close collaboration with the Institute of Physics of SAS, Bratislava, Slovakia and other PALS groups (Poland) and the internal probing ones such as differential scanning calorimetry (DSC) and broadband dielectric spectroscopy (BDS) in close co-operation with the top dielectric teams in Europe (Germany, Spain) as well as on continuously developed phenomenological and theoretical methodologies of the microscopic characterization of the small molecular, oligo- and poly-meric organics compounds.
Materials, techniques, methodologies:
Our systematic fundamental research is aimed on two basic groups of organic materials: 1) pure organic compounds such as small molecular and oligo- and polymer media and 2) the so-called organic–inorganic nanosystems such as organic media confined in inorganic matrices (confiners) and organic media in nanocomposites with inorganic compounds (fillers). Various organic compounds of different types of the chemical structure such as non-polar vdW-bonded media and polar non-H-bonded (aprotic) and H-bonded (protic) ones are investigated by means of two special external microscopic probes: molecular-sized spin probes via ESR and atomic-sized ortho-positronium via PALS. Molecular mobility of one of the smallest quasi-spherical spin probe, namely, 2,2,6,6-tetra-methyl-piperidinyl-1-oxy (TEMPO), reflects the local microstructural and microdynamical surroundings of the investigated organic medium being measured through the spectral parameter of mobility, 2Azz‘, and the reorientation correlation time, τc, over a wide investigated temperature range from 100 K up to 360 K. The microscopic changes in the spin probe dynamics at the characteristic ESR and/or PALS temperatures are related to and interpreted by means of the changes in the specific heat and the electric dipole reorientation as obtained by DSC or BDS, respectively.
The representative results:
The first type of the problems consists in a unique combination of ESR and PALS studies on various pure organic materials> in the bulk state. Fig.1 displays the ESR response of the spin system 1,4-poly(isoprene)/TEMPO in a form of the temperature dependence of the spectral parameter of mobility which exhibits several distinct regions of spin probe mobility defining a set of the characteristic ESR temperatures such as TXislow, T50G and TXifast.
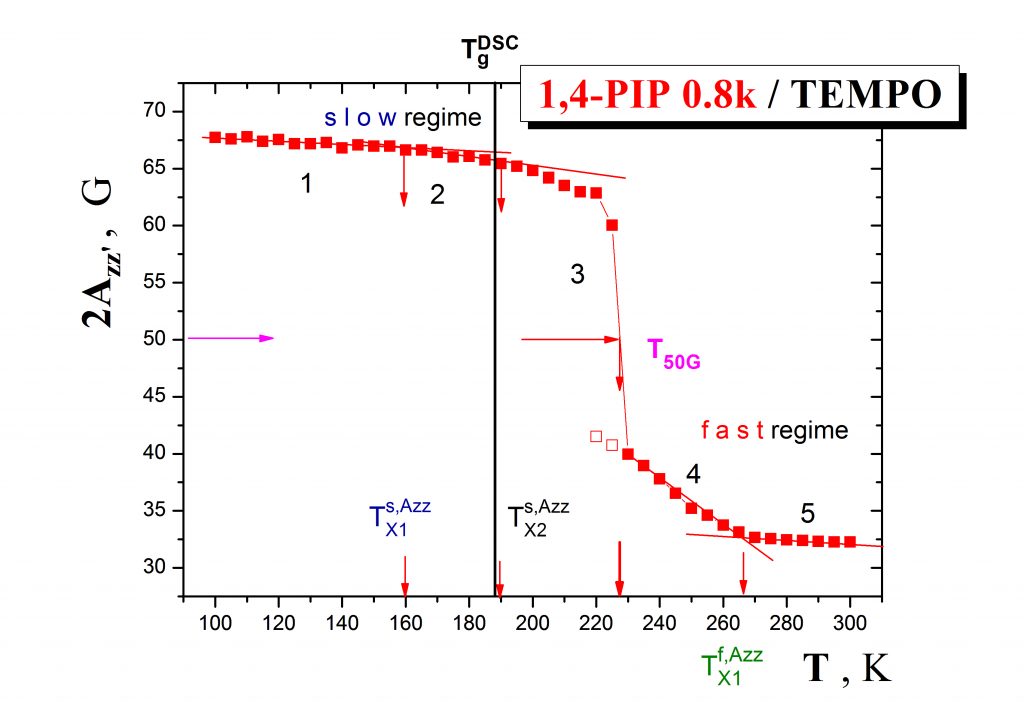
Fig.2 shows the more detailed ESR response of the spin probe TEMPO in oligomeric 1,4-poly (isoprene) in terms of the correlation time as a function of temperature which demonstrates the co-existence of slow and fast moving spin probe molecules over a certain temperature range, i.e., the occurence of the dynamic heterogeneity phenomenon even in one-component amorphous organic medium.This effect is of great importance in the field of glassy and polymer physics for understanding the evolution of molecular motion in glass-forming compounds on going from the normal liquid state through supercooled liquid state to the glassy state und the closely related glassification phenomenon as the basic feature of amorphous substances.
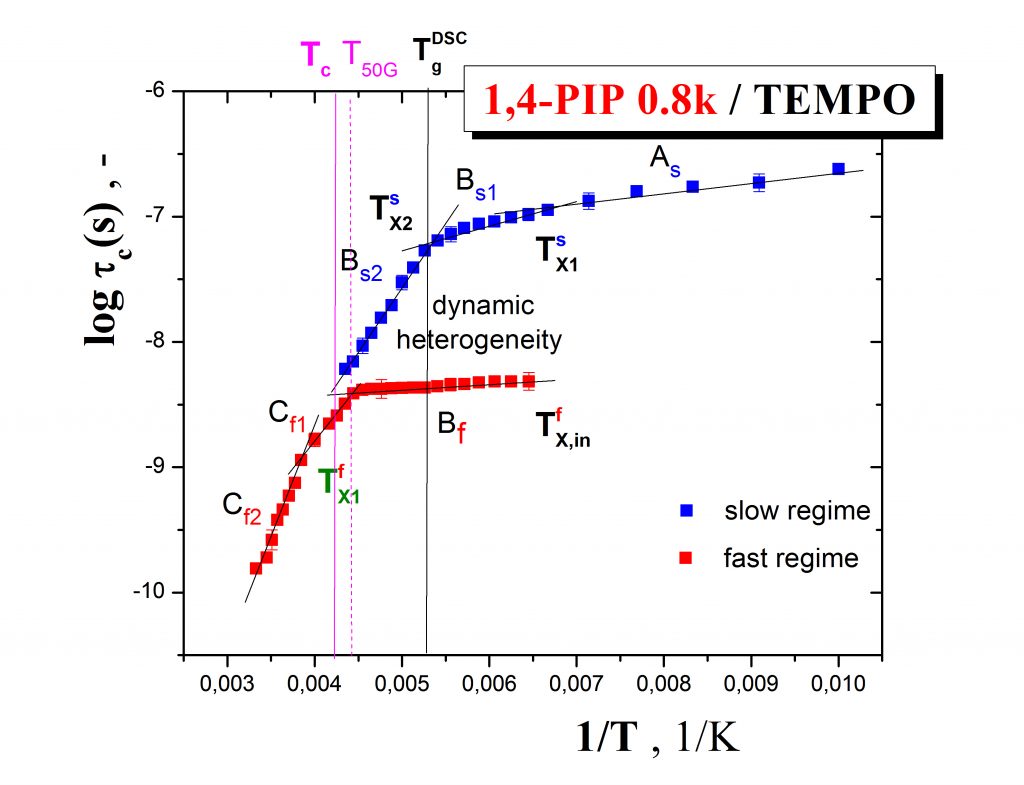
Annihilation of ortho-positronium (o-Ps) probe expressed by the temperature dependence of the o-Ps lifetime, τ3, mirrors the local free volume microstructure and its change due to the local dynamics of constituents in non-conducting materials such as organic media or/and inorganic matrices. Typical PALS response exhibits a few regions described by the characteristic PALS temperatures: Tb1G, Tb2G, TgPALS and Tb1L, Tb2L as demonstrated on the case of the 1,4-PIP sample – Fig.3. In some cases close coincindencies between the characteristic ESR and PALS temperatures can be found which indicate the common origin of both the related changes in the spin probe TEMPO rotation mobility and the o-Ps annihilation.
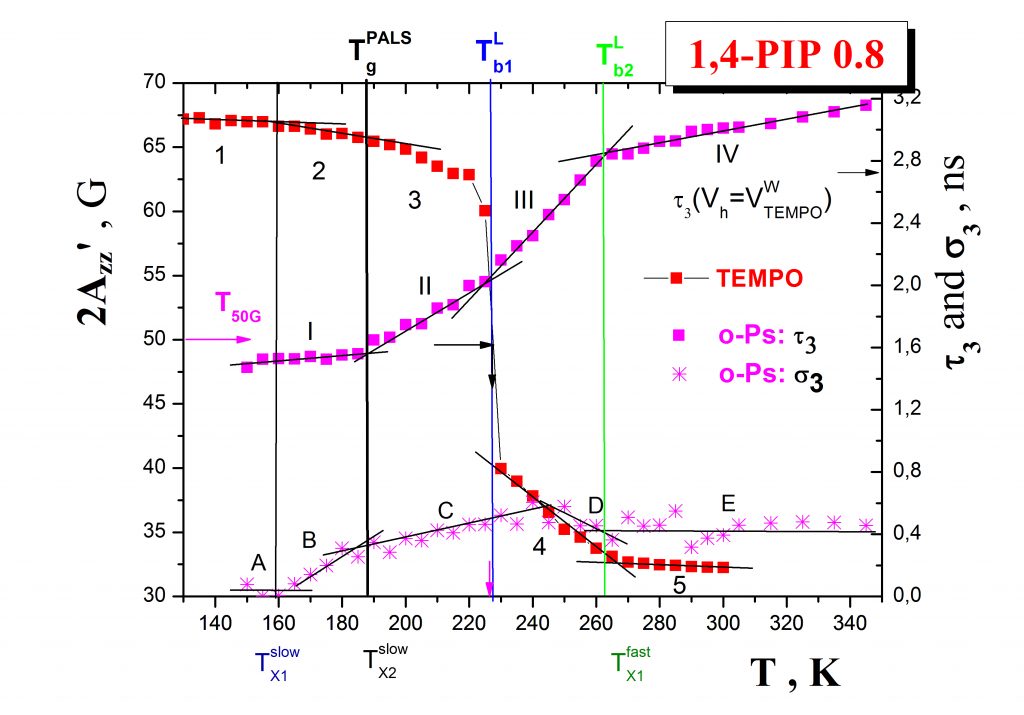
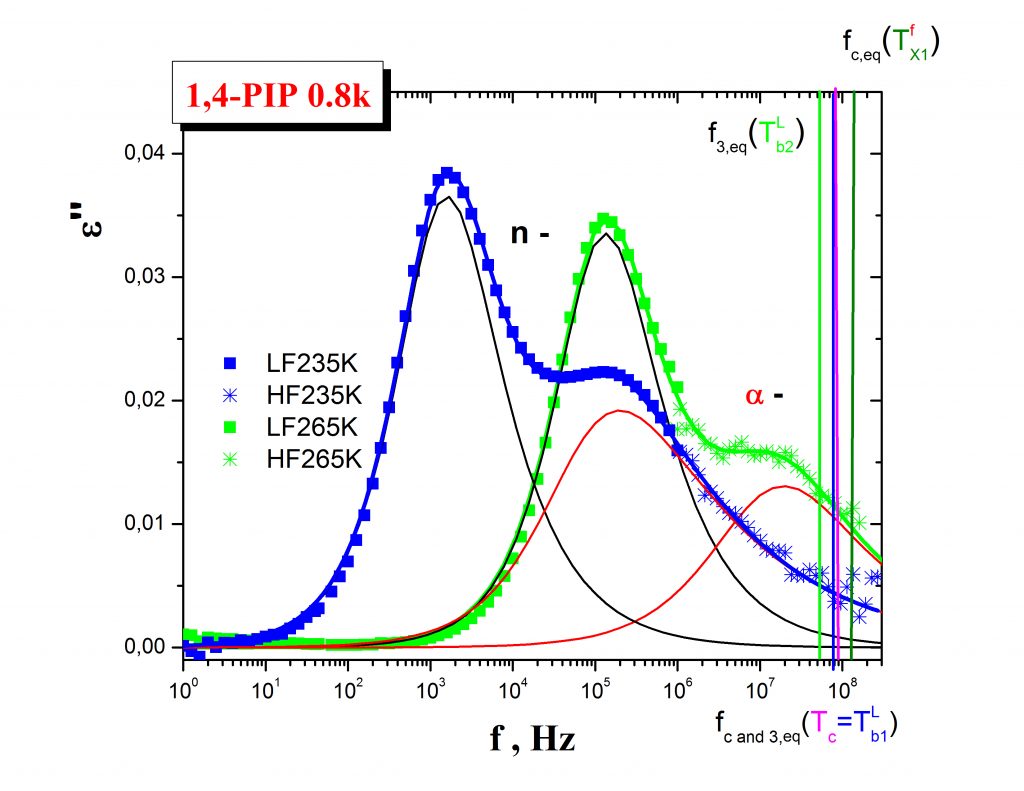
The mutual relationships between these ESR and PALS crossover effecs and, in some cases, the common origins of their coincidencies are currently revealed by using an appropriate combination of internal probe techniques such as DSC and/or BDS. Thus, in our representative case of 1,4-PIP Fig. 4 shows that the coincidence between the slow to fast motion regime transition of molecular probe at T50G and the change in the free volume expansion in liquid state at Tb1L are related to the onset of the local segmental α- relaxation process in the medium. On the other hand, the coincidence between an acceleration in the mobility within the fast regime at TXifast and the quasi-saturation effect in o-Ps lifetime at Tb2L are found to be close to the maximum of the primary (α-) relaxation, i.e., local segmental process as well as to the onset of the secondary normal (n-) relaxation, i.e., global chain process.
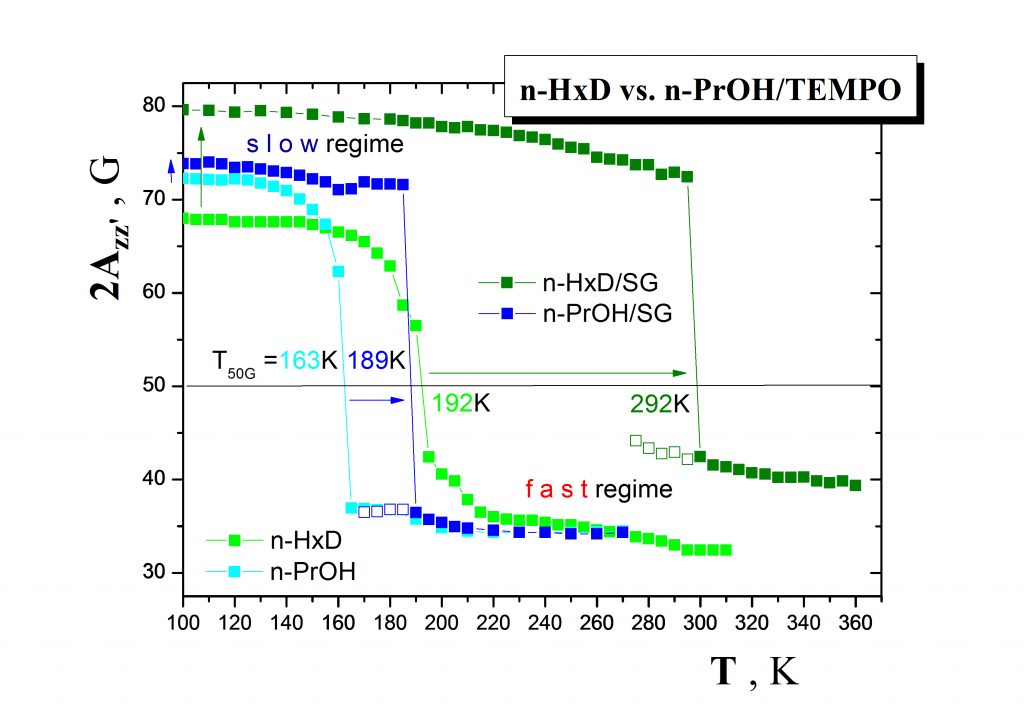
The second type of the problems solved in our research group consists in search for utilization of the afore-mentioned external probes in the microscopic characterization of various nanostructured systems and in development of the corresponding characterization methodologies. As an example, one group of them includes various organic media such as small molecular and oligomeric compounds confined in a variety of inorganic matrices (confiners). Fig.5 demonstrates the ESR responses of the spin probe TEMPO in a typical non-polar medium n-hexadecane (n-HXD) in comparison with a typical polar protic one, i.e. n-propanol (n-PrOH) in both the bulk states and the confined states in the inorganic matrix of silicagel (SG). The observed dramatic differences reflect the delicate mutual interactions between all the three components of the studied systems and suggest the potential of the molecular spin probing method in the detailed structural-dynamic characterization of the various confined organic media.
Recent references
- BARTOŠ, Josef – ŠVAJDLENKOVÁ, Helena – ŠAUŠA, Ondrej – LUKEŠOVÁ, Miroslava – EHLERS, D. – MICHL, M. – LUNKENHEIMER, P. – LOIDL, A. Molecular probe dynamics and free volume in organic glass-formers and their relationships to structural relaxation: 1-propanol. In Journal of Physics- Condensed Matter, 2016, vol. 28, no. 1, 015101.
- ŠVAJDLENKOVÁ, Helena – ZGARDZINSKA, B. – LUKEŠOVÁ, Miroslava – BARTOŠ, Josef. Spin probe dynamics in relation to free volume in crystalline organics from ESR and PALS: Cyclohexane. In Chemical Physics Letters, 2016, vol. 643, p. 98-102.
- LUKEŠOVÁ, Miroslava – ZGARDZINSKA, B. – ŠVAJDLENKOVÁ, Helena – ZALESKI, R. – CHARMAS, B. – BARTOŠ, Josef. Spin probe dynamics in relation to free volume in crystalline organics from ESR and PALS: N-tridecane. In Physica B: Condensed Matter, 2015, vol. 476, p. 100-108.
- LUKEŠOVÁ, Miroslava – ŠVAJDLENKOVÁ, Helena – SIPPEL, Pit – MACOVÁ, Eva – BEREK, Dušan – LOIDL, Alois – BARTOŠ, Josef. Spin probe dynamics of n-hexadecane in confined geometry. In European Physical Journal B, 2015, vol. 88, art.no. 46.
- BARTOŠ, Josef – ŠVAJDLENKOVÁ, Helena – LUKEŠOVÁ, Miroslava – YU, Y. – KRAUSE-REHBERG, R. Molecular dynamics and free volume in organic glass-formers: A series of oligomer and polymer 1,4-poly(isoprene)s. In Chemical Physics Letters, 2014, vol. 602, p. 28-33.
- BARTOŠ, Josef – ŠVAJDLENKOVÁ, Helena – ZALESKI, R. – EDELMANN, M. – LUKEŠOVÁ, Miroslava. Spin probe dynamics in relation to free volume in crystalline organics by means of ESR and PALS: n-Hexadecane. In Physica B: Condensed Matter, 2013, vol. 430, p. 99 – 105.
- BARTOŠ, Josef – ŠVAJDLENKOVÁ, Helena – YU, Y. – DLUBEK, G. – KRAUSE-REHBERG, R. Molecular probe dynamics and free volume in glass-formers: 1,2- and 1,4-poly(butadiene)s. In Chemical Physics Letters, 2013, vol. 584, p. 88 – 92.
- YANG, Yu – DLUBEK, Gunter – BARTOŠ, Josef – ŠVAJDLENKOVÁ, Helena – KRAUSE-REHBERG, Reinhard. Relationships between positron lifetime and dynamics of polymers. In Materials Science Forum, 2013, vol. 733, p. 179 – 182.
- BARTOŠ, Josef – ISKROVÁ – MIKLOŠOVIČOVÁ, Martina – CANGIALOSI, D. – ALEGRÍA, A. – ŠAUŠA, Ondrej – ŠVAJDLENKOVÁ, Helena – ARBE, A. – KRIŠTIAK, Jozef – COLMENERO, J. Positron annihilation and relaxation dynamics from dielectric spectroscopy: Poly (vinylmethylether). In Journal of Physics: Condensed Matter, 2012, vol. 24, art.no.155104.
- ŠVAJDLENKOVÁ, Helena – ŠAUŠA, Ondrej – ISKROVÁ – MIKLOŠOVIČOVÁ, Martina – MAJERNÍK, V. – KRIŠTIAK, Jozef – BARTOŠ, Josef. On the relationships between guest molecular dynamics and free volume in a series of small molecular and polymer glass-formers. In Chemical Physics Letters, 2012, vol. 539 – 540, p. 39 – 44.
- BARTOŠ, Josef – ISKROVÁ, Martina – KOHLER, M. – WEHN, R. – ŠAUŠA, Ondrej – LUNKENHEIMER, P. – KRIŠTIAK, Jozef – LOIDL, A. Positron annihilation response and broadband dielectric spectroscopy: Salol. In European Physical Journal E : Soft Matter and Biological Physics, 2011, vol. 34, p. 104 – 114.
- BARTOŠ, Josef – ŠAUŠA, Ondrej – KOHLER, M. – ŠVAJDLENKOVÁ, Helena – LUNKENHEIMER, P. – KRIŠTIAK, Jozef – LOIDL, A. Positron annihilation and broadband dielectric spectroscopy : A series of propylene glycols. In Journal of Non-Crystalline Solids, 2011, vol. 357, p. 376 – 384.
- BARTOŠ, Josef – ŠAUŠA, Ondrej – SCHWARTZ, G.A. – ALEGRÍA, A. – ALBERDI, J.M. – ARBE, A. – KRIŠTIAK, Jozef – COLMENERO, J. Positron annihilation and relaxation dynamics from dielectric spectroscopy and nuclear magnetic resonance: cis-trans-1,4-poly(butadiene). In Journal of Chemical Physics, 2011, vol.134, art.no.164507 p.1-10.
- ŠVAJDLENKOVÁ, Helena – ISKROVÁ, Martina – ŠAUŠA, Ondrej – DLUBEK, G. – KRIŠTIAK, Jozef – BARTOŠ, Josef. The spin probe dynamics and the free volume in a series of amorphous polymer glass-formers. In Macromolecular Symposia, 2011, vol. 305, p. 108 – 115.

 kontakty
kontakty
